
We provide competent answers to your questions.
Understanding your business processes and knowledge about the regulatory requirements is a must for development of an optimized Clinical Product Lifecyle strategy. 15+ years experience enables us to provide you with support you need. We carefully consider your ideas for product development, indications, claims but also market, while product prioritisation with portfolio analysis, market research or competitors analysis is performed.
GAP-analyse fehlt.
Unterschied Consulting/Workshop muss klarer werden.
Consulting: Wenige (1-3) individuell Teilnehmer, seitens Kunde.
Workshop: Viele Teilnehmer / teils Frontalveranstaltung, teils Arbeitspakete in Gruppen bearbeitend.
We support you in the developement of a clinical-regulatory strategy, including optimisation of your processes and product pipeline. Together we analyse:
…business development
…optimisation of your product portfolio and product life cycles
…implementation of the MDR
…definition of appropriate clinical targets/aims

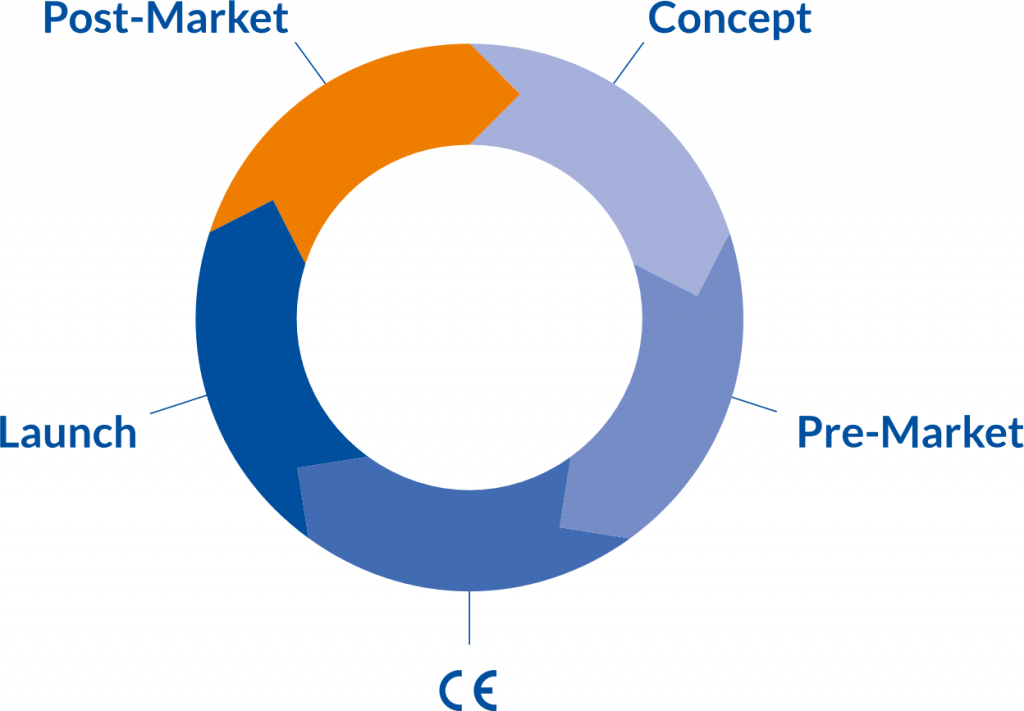
Clinical Product Lifecycle
Constant technological & digital innovations together with more stringent compliance requirements as the MDR (EU) 2017/745 characterize the medical device industry of today. Companies face enormous challenges. The MDR (EU) 2017/745 is strengthening a professional “clinical” product lifecycle. Product innovations need an early, clear and lean “clinical approach”. In order to be successful, marketing aspects and claims must be taken into account. Regarding clinical evaluation it is necessary to establish a clinical development plan as it is addressed in Annex XIV of the MDR, “indicating progression from exploratory investigations, such as first-in-man studies, feasibility and pilot studies, to confirmatory investigations such as pivotal clinical investigations and a PMCF with an indication of milestones and a description of potential acceptance criteria.”
We provide efficient and responsive strategic consultancy services to implement a clinical product lifecycle strategy.
Why?
An in depth analysis supports you in the decision-making, which e.g. results in a clinical-regulatory strategy. It identifies tangible measures, ranging from targeted training of your employees and study staff to CEP implementation, and definition of the development pipeline, including its marketing and funding plan.

The effective identification and implementation of your measures can involve the board of directors, management, investors, as well as management from research and development, marketing and quality management.


